The majority of studies submitted at ASU do not need to be reviewed by the full Institutional Review Board. Studies that may require full committee review are generally those that may have greater than minimal risk or are working with vulnerable populations. When working with these types of studies, it is important to keep in mind the submission deadlines if there is a time constraint in carrying out the study:
Bioscience IRB
| Meeting date | Submission deadline |
|---|---|
| December 17, 2025 | December 1, 2025 |
| January 14, 2026 | December 29, 2025 |
| February 11, 2026 | January 26, 2026 |
| March 18, 2026 | March 2, 2026 |
| April 15, 2026 | March 30, 2026 |
| May 20, 2026 | May 4, 2026 |
| June 17, 2026 | June 1, 2026 |
| July 15, 2026 | June 29, 2026 |
| August 19, 2026 | August 3, 2026 |
Social behavioral IRB
| Meeting date | Submission deadline |
|---|---|
| November 21, 2025 | November 5, 2025 |
| December 19, 2025 | December 3, 2025 |
| January 16, 2026 | December 31, 2025 |
| February 20, 2026 | February 4, 2026 |
| March 20, 2026 | March 4, 2026 |
| April 17, 2026 | April 1, 2026 |
| May 15, 2026 | April 29, 2026 |
| June 19, 2026 | June 3, 2026 |
Prepare materials
Before beginning a study in ASU’s electronic submission system (ERA), confirm the following items have been done:
- Identified the principal investigator (cannot be a student).
- Completed a protocol template.
- Completed consent forms or cover letters.
- Completed assent forms and parental permission forms (if your project involves minors).
- Completed copies of recruitment materials and study instruments.
- Identified the study team and had them complete CITI Training.
- Reviewed the special considerations to see if any of the topics apply to the study.
- Gathered grant submission/other funding documents for upload in the event the project is funded.
- Obtained site permissions, if needed.
Protocol templates
Every submission must include one of the two protocol templates. Fill out every section of the form according to the guidance provided within it. If a section does not apply, provide a brief explanation as to why.
- Form: Social Behavioral Protocol — research studying behavior, opinion, social history and educational practice.
- Form: Bioscience Protocol — research involving invasive procedures, exercise, dietary manipulation, human physiology and effectiveness of medical products.
Consent forms
Almost every study will require a consent form to be developed and attached to the submission. Use the appropriate form for guidance:
- Short Form Consent Form for studies that are extremely low risk including most surveys, interviews, and observations.
- Social and Behavioral Long-Form Consent Form for studies with moderate risks involving social and behavioral procedures.
- Bioscience Long-Form Consent Form for studies with moderate risks involving bioscience procedures.
If the research involves minors, consult Research Involving Minors or Children for relevant consent, assent and permission form templates. For medical release forms, see Medical Records and HIPAA.
Recruitment materials
You must submit any recruitment materials to be used for review and approval. You may create your own or use one of the following templates for guidance:
- Template: Social Media/Email/Text Message Recruitment.
- Template: Recruitment Script.
Site permissions
For projects taking place in a non-public or non-ASU setting (for example, a middle school classroom, a local organization or a clinic), you should obtain site permission. Contact the site to determine their policies for obtaining permission.
Special consideration: Translating documents and materials
Translated documents should not be included in the initial IRB submission as there may be changes made to the materials during review. The initial submission should include English versions of all documents and the protocol should explain what languages will be used in the research. The reviewer will request translated materials after the English versions of the documents have been approved. A completed Translation Certification form will need to be submitted with translated materials. The form outlines the translation process. Translators can be anyone sufficiently fluent in the language.
Special consideration: Another IRB is reviewing the study
When another institution’s IRB or a central IRB (such as WIRB) will be acting as the IRB of record, the submission will undergo a Local Context Review. Contact the ASU IRB before proceeding. A local context review requires different forms and materials to be submitted. For more information, visit Collaborating with Other Institutions: Affiliation Agreements and Review Procedures.
Special consideration: Multiphase studies
If the project will include multiple phases, some of which are not finalized, the researcher should submit for the phases that have been finalized. When a new phase is ready to be implemented, you can submit either a modification to the approved study or a new study.
Submitting the study to the IRB
Use our step-by-step tutorial or follow the steps below:
1. Log into ERA using ASURITE ID and password.
Select “Create New Study”.
Fill in the fields as appropriate. Upload a completed Template: Social Behavioral Protocol OR Template: Bioscience Protocol to the Attach the Protocol section.
In the Consent Forms and Recruitment Materials section, attach any of the following as applicable:
- Consent Form(s).
- Assent Form(s).
- Parental Consent Form(s).
- Recruitment Script(s).
In the Supporting Documents section, attach any of the following as applicable:
- Surveys.
- Questionnaires.
- Interview Questions.
- Instruments.
- Other Documents.
When ready to submit the ERA application, the principal investigator will need to click the “submit” button, which is only viewable to the submitter.
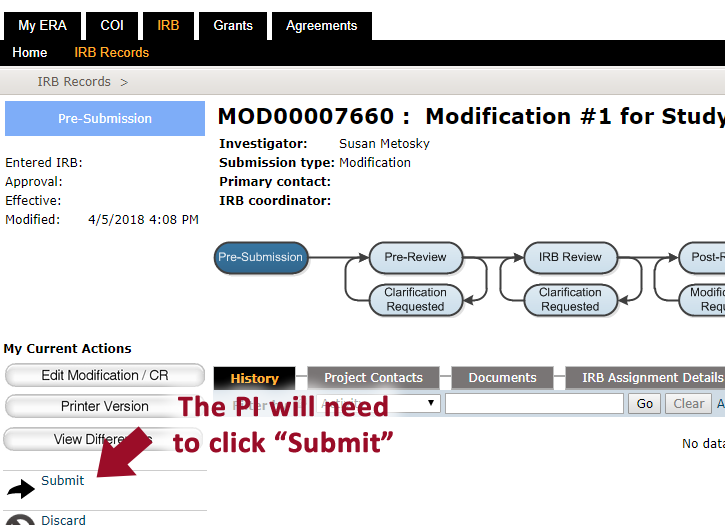
Confirming the study has been submitted for review
Check that the study is in the “Pre-Review” state and that “Submitted” is the most recent event. If the study shows that it is still in the “Pre-Submission” state, it is likely that the principal investigator still needs to review and “submit”.
Need to find your study?
Use our step-by-step tutorial.