NOTICE (01.11.2024)
ASU’s Institutional Review Board has developed a FAQ document to assist researchers with commonly asked IRB-related questions. Researchers are encouraged to review this document and may contact [email protected] if additional clarification is needed.
NOTICE (12.19.2022)
ASU’s IRB has implemented a Wizard tool to streamline the IRB review process for studies that fall under one or more exempt categories. ASU researchers can gather more information about the Wizard tool in the link below to see if their study is eligible for a determination through the Wizard tool and the next steps.
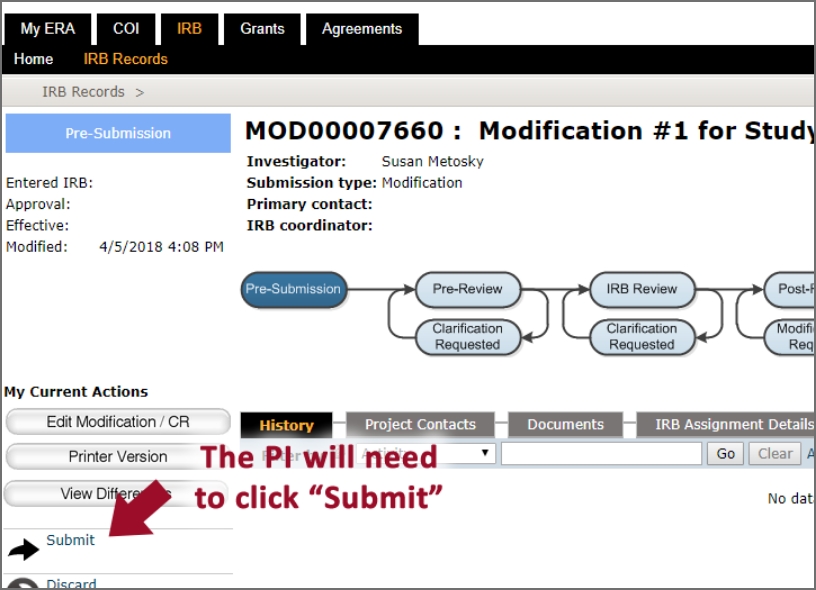
How to submit to the IRB?
If you would like to watch some walkthrough videos, please click on the link below.
All ASU and research-related projects involving humans as subjects must be reviewed and approved by ASU’s Institutional Review Board prior to implementing studies, including recruitment and screening activities. The role of the IRB is to review all proposed research involving human subjects to ensure that subjects are treated ethically and that their rights and welfare are adequately protected.
All institutions engaged in human subjects research that is not exempt from 45CFR46, and is conducted or supported by any HHS agency must be covered by an Office for Human Research Protections-approved assurance of compliance. The Federalwide Assurance is the only type of assurance accepted and approved by OHRP. The Assurance is a formal declaration to HHS that it will comply with the requirements set forth in 45CFR46, as well as the terms of Assurance.
Copy code to clipboardWhat activities require IRB review?
IRB review is required if the study involves human subjects and meets the definition of research.
A human subject is defined as “a living individual about whom an investigator conducting research obtains data through intervention or interaction with the individual or identifiable private information.” This definition can include online surveys, collection of data from Twitter, analysis of coursework, interviews, etc.
Research is defined as “a systematic investigation, including research development, testing and evaluation, designed to develop or contribute to generalizable knowledge.”
If you are unsure whether your project meets this definition and requires review, e-mail us with a brief description of the study and specific direction will be provided.
Special case: Research as part of coursework
Some student classroom projects may not be considered research (and might not require IRB review) if they meet all three of the following criteria:
- Procedures are low-risk or no-risk.
- The data will not be used, published or otherwise disseminated outside of the classroom.
- The objective is to provide students with training about and experience with research methods.
For more information, review the Guidance: Classroom research.
Special case: Collaborating with other universities or institutions
See Collaborating with other institutions: Affiliation agreements and review procedures.
Special case: ASU as a study site
If ASU is serving as a study site and no ASU students, faculty, staff or employees will be members of the research team (or will be collaborating with the research team besides being subjects of the research), then formal review by the ASU IRB is not required. To note, the ASU IRB cannot provide access to the study sample. You may be required to contact the department/unit (or the Provost Committee for broad recruitment of ASU students) for additional approval.
Submitting for review
Submit your application through ERA, ASU’s online submission system. See the Protocol submission page for more information.
The New Common Rule — What does this mean for ASU Researchers?
This briefing is intended to aid researchers with understanding the New Common Rule and how it will be implemented by the Arizona State University Institutional Review Boards. This is not a complete list of all changes in the regulation but it covers areas of common interests and it highlights important areas.
A few of the changes implemented should result in a reduction of burden for investigators and others present additional work for investigators and the IRB.