Modifications
All modifications to studies approved as Expedited and Full Board must be submitted for review and approval. For studies with exempt approval, please email [email protected] for guidance on submitting a modification.
Modifications can include:
- Adding study team members.
- Changing the wording of a consent form.
- Adding a study site or new recruitment method.
- Adding a new phase of the study.
- Adding or removing survey questions.
Submit all modifications through the Enterprise Research Administration system. The submission, review and approval process is similar to an initial study. Many modifications will require revised protocol documents, consent forms, and other study materials to be uploaded to ERA.
Studies approved as Exempt require modifications to be approved by the IRB which have the potential to change the review category to be submitted.
If you have questions about whether or not a modification should be submitted, please contact us.
Need to find your study?
Use our step-by-step tutorial.
Create a modification
Use our step-by-step tutorial or follow the steps below:
1. Log into ERA using ASURITE user ID and password.
2. Click “IRB”.
3. Click “IRB records”.
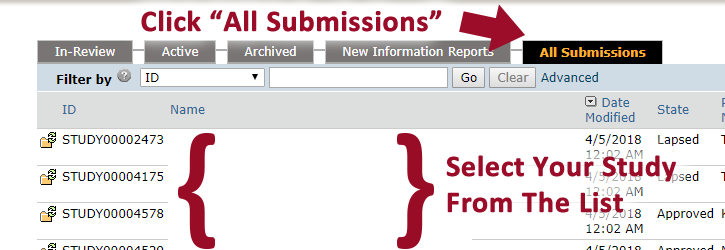
4. Click “All Submissions”.
5. Click on the study title you wish to modify.
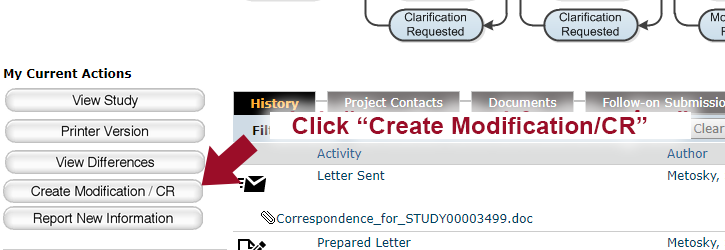
6. Click “Create Modification/CR”.
7. Select “Modification”* under “What is the Purpose of this submission?” *Note: “Continuing Review” and “Modification and Continuing Review” should *ONLY* be used if your study is up for continuing review.
8. Choose your modification scope. Select one or both depending on what you need to change.
9. Press “Continue” and complete each section.
10. If you are uploading revised documents, use the “Update” option. If you are adding new documents, use the “Add” option.
11. When all changes have be made, the PI will need to click “Submit”** to send the study to the IRB **Note: Only the PI can SUBMIT an application. If you are not the PI, you will not see the Submit button.
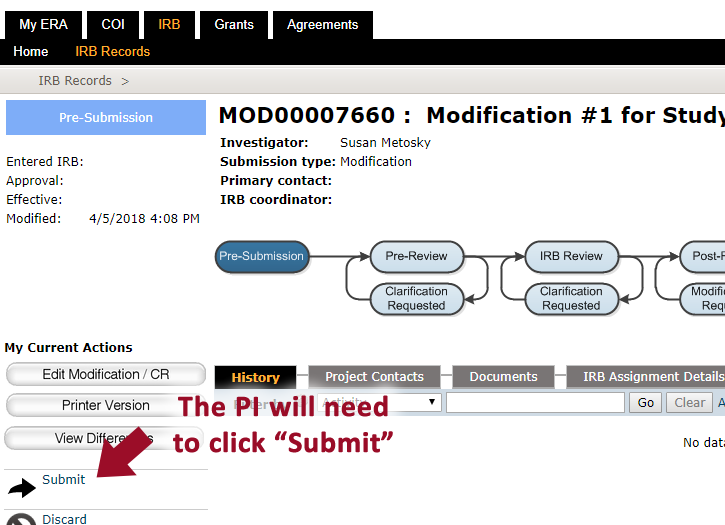
12. After the study is submitted, confirm that it is in the “Pre-review” state.
Continuing review
Studies approved as Expedited and Full Board require a yearly Continuing Review. You will receive a notification 3 months prior to the deadline for a Continuing Review. Investigators must meet required Continuing Review dates. If an investigator fails to submit a continuing review or the IRB does not approve a continuation before the date of expiration, all research activities must stop, including:
- Subject recruitment or enrollment.
- Collection of data/information.
- All research-related interventions or interactions with currently enrolled subjects.
- Data analyses involving subject-identifiable data.
Continuing Reviews are submitted through ERA. The application requires answers to several questions and an updated progress report. This progress report should include:
- The number of participants.
- A summary of any:
- Changes to the research approved by the IRB since the IRB’s initial review or the last continuing review.
- Unanticipated events.
- Subjects who withdrew from the project and the reasons for withdrawal, if known.
- Complaints about the research from subjects or others since the last IRB review.
- Any new and relevant information, published or unpublished, since the last IRB review, especially information about risks
If the study was approved as Full Board, it will likely require a Full Board Continuing Review. The Continuing Review application must be submitted at least 2 weeks before an IRB meeting date. If a Continuing Review requiring Full Board review is not received in time, work on the project will need to cease and it will need to be reviewed at the next meeting.
Submit a Continuing Review
1. Log into ERA using ASURITE user ID and password.
2. Click “IRB”.
3. Click “IRB records”.
4. Click “All Submissions”.
5. Click on the study title requiring a Continuing Review.
6. Click “Create Modification/CR”.
7. Select “Continuing Review”.
8. Fill out the form following the instructions on the form.
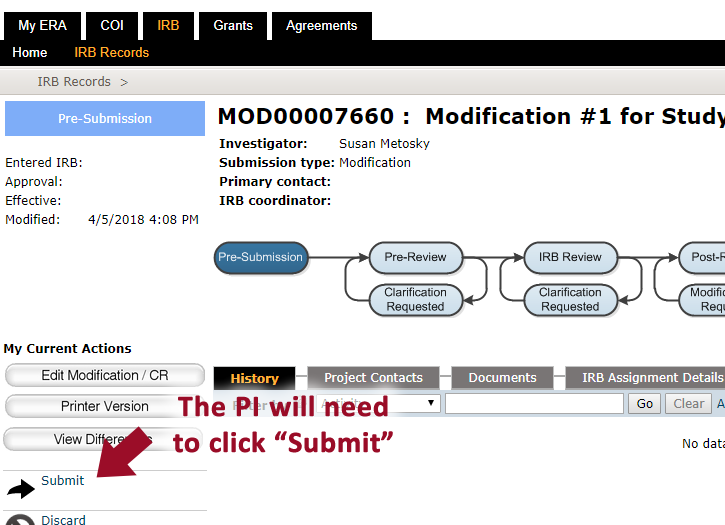
9. When all changes have be made, the PI will need to click “Submit”** to send the study to the IRB [**Note: Only the PI can SUBMIT an application.] If you are not the PI, you will not see the Submit button.
Close a study
When research activity on a study has been completed, the study must be closed. Research activity is defined as any of the following:
- Subject recruitment or enrollment.
- Collection of data/information from or about living individuals.
- All research-related interventions or interactions with currently enrolled subjects*.
- Data analyses involving subject identifiable data.
Close a study
1. Log into ERA using ASURITE user ID and password.
2. Click “IRB”.
3. Click “IRB records”.
4. Click “All Submissions”.
5. Click on the study title you wish to close.
6. Click “Create Modification/CR”. This option will allow you to close a study.
7. Select “Continuing Review”.
8. Checking the first four Research Milestones in the continuing review will trigger a study closure.
9. Complete the rest of the information including attaching a summary of findings (usually one to four paragraphs).
10. When all information has been filled in, the PI will need to click “Submit”** to send the closure to the IRB [**Note: Only the PI can SUBMIT a study closure. If you are not the PI, you will not see the Submit button].
11. After the closure has been submitted, confirm that it is in the “Pre-Review” state.