What is reportable new information?
During a research study, any adverse events, unanticipated problems involving risk, and non-compliance must be reported to the IRB. The guidance contained here is intended to help ensure that the reporting and review of these events occur in a timely, meaningful way so that research participants can be protected from avoidable harms. Any serious events must be reported within 24 hours. Non-serious adverse events must be reported within 5 business days. Serious events include, but are not limited to:
- Death
- Life-threatening event
- In-patient hospitalization
- Prolongation of existing hospitalization
- A persistent or significant disability/incapacity
What must be reported?
The following information must be reported:
- Protocol violation that harmed subjects or others or that indicates subjects or others might be at increased risk of harm.
- Information that indicates a new or increased risk, or a safety issue. For example, new information (e.g., a safety monitoring report, publication in the literature, or investigator finding) indicates an increase in the frequency or magnitude of a previously known risk, or uncovers a new risk.
- Complaint of a subject that indicates subjects or others might be at increased risk of harm or at risk of a new harm.
- Complaint of a subject that cannot be resolved by the research team.
- Any changes significantly affecting the conduct of the research.
- Any harm experienced by a research subject or other individual(s) that, in the opinion of the investigator, is unexpected and at least probably related to the research procedures.
- Non-compliance with the federal regulations governing human research or with the requirements or determinations of the IRB.
- Failure to follow the protocol due to the action or inaction of the investigator or research staff.
- Breach of confidentiality.
- Change to the protocol taken without prior IRB review to eliminate an apparent immediate hazard to a subject.
- Incarceration of a subject in a study not approved by the IRB to involve prisoners.
- Premature suspension or termination of the research by the sponsor, investigator or institution.
How do I submit a reportable event?
Report an event using the “Reportable New Information” button in ERA. The system will ask several questions and prompt you for a brief description of what happened. To report new information about a study:
1. Log into http://era.oked.asu.edu/.
2. Click “IRB”.
3. Click “IRB records”.
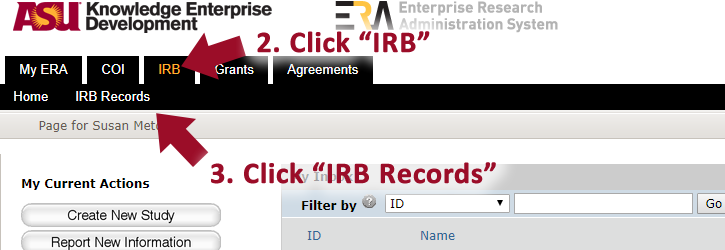
4. Click “All Submissions”.
5. Click on the study title requiring an RNI (Reportable New Information).
6. Click “Report New Information”.
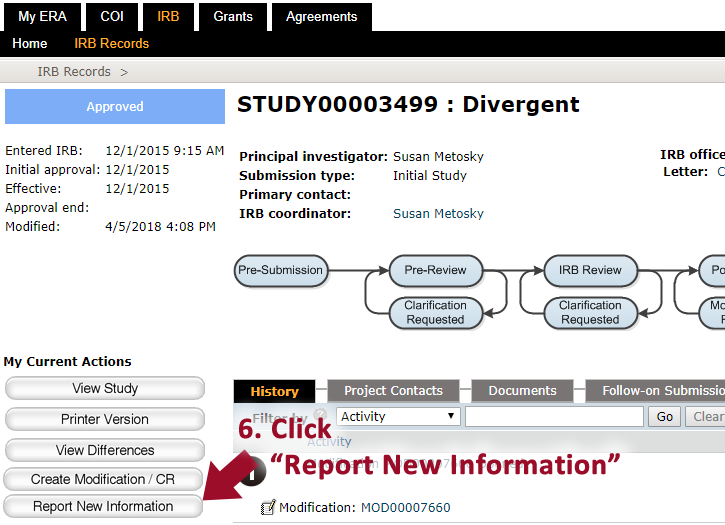
7. Fill in the appropriate details. If you are unsure of what to select, contact us.
8. Once you are finished with all the details, click “Continue” and click “Submit RNI”.
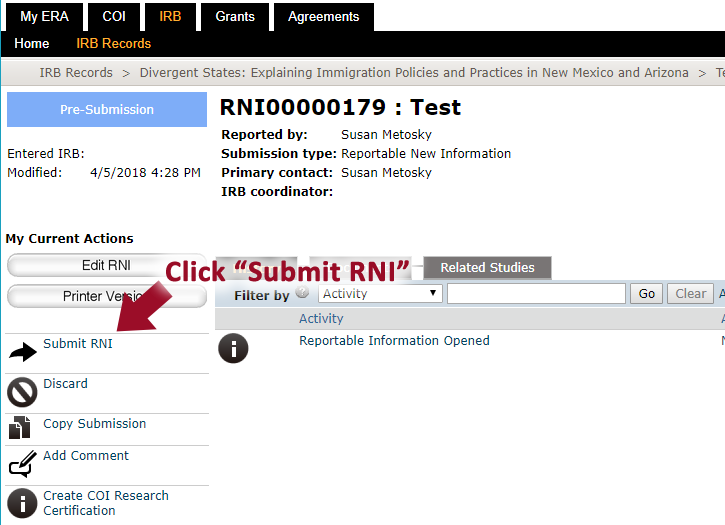
9. After the RNI has been submitted, it will say “RNI Submitted” in the “History” tab.